I think the week we did the Enzyme Lab was the most confused I have ever been in Biology. I have been putting this blog off for awhile but I know my grade depends on getting it done, so here it is.
Our group had a simple setup. (or so Michael said, didn't seem that simple to me) We mixed 3 ml of water with 3 ml of hydrogen peroxide. We added the enzyme yeast to make a chemical reaction that would make oxygen. The yeast, our enzyme, breaks the oxygen from the hydrogen peroxide and releases the oxygen into the air. Using a pressure probe we measured the pressure coming from the chemical reaction. We did our experiment three different ways. In the first we changed the amount of enzyme we put in. In the second we changed the temperature of the solution that we put the enzyme in to make the reaction. In the third experiment we changed the water in the solution to a pH solution. We did each experiment 3 times so we could calculate accurate data for each experiment.
Here are the different graphs we used to collect our data from the experiment.
In this graph we changed the amount of enzyme that we added to the solution. Or a simpler way to say it!...We changed the amount of drops we added to the mixture in the test tube. (10, 20, 30, or 40 drops.) The graph has a constant slope. I'm going to say what this means in two ways.
SIMPLE WAY: The more enzyme thats in the solution the more pressure there is.
SMART WAY: The speed at which the reaction occurs is a direct result of the ratio of the enzyme to the solution. (thank you Michael for helping me come up with that! =])
In this graph we changed the temperature of the solution where the reaction is taking place. The simple explanation....We changed the temperature of the stuff in the test tube. (0, 25, 38, or 80 degrees Celsius.) We kept the yeast at 25 drops every time.
From this graph we noted that freezing the solution at 0 degrees Celsius and heating it up to 80 degrees Celsius was not good on the reaction. When the temperature got to hot the graph decreased rapidly. This is because when there is to much heat is causes the enzymes to become denatured. (FUN FACT: Denatured means the shape changed...you know you can learn a lot looking at other peoples blogs.) We found out the best temperature for making oxygen was between 25 and 38 degrees Celsius. This is where the highest rate of reaction occurred.
Ok this was the part in the experiment that I was really confused. So I decided to look at my fellow group members blogs( Michael and Kyle H) to try and understand what went on better. Well....that made me more confused. Kyle said that the graph was messed up because Sierra(She was also in our group) couldn't measure out drops right....So there was one thing they had in common so I am going to talk about that.
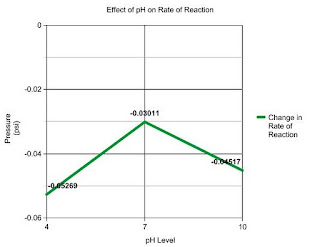
In this graph we changed the 3ml of water in the solution to 3 ml of different pH solutions. (4, 7, and 10). So I think the graph kind of shows this but we found that the most oxygen was produced at the pH level 7. (FUN FACT: which is normal by the way!) It produced less oxygen at the pH levels 4 and 10.
In my research on enzymes this is something interesting I learned: Superoxide Dismutase, or SOD, is a metalloenzyme. SOD plays an extremely important role in the protection of all life-systems. The enzyme superoxide dismutase, or SOD, catalyzes into oxygen and hydrogen peroxide. SOD is present in essentially every cell in the body. The two major forms of superoxide dismutase (SOD) in humans are the mitochondrial manganese SOD and the cytosolic copper/zinc SOD. When organisms cause serious disease, it takes the body a very long time to recover, and depending on the strength of the patient the bacteria may win. The ability of the enzyme to provide some protection to organisms is shown by the existence of a motor neuron disease in individuals who have point mutations in SOD. The absence of SOD may lead to a form of anemia. A number of tumour cells have been found to be deficient in SOD. The absence of SOD activity seems to support cancer. In addition, SOD mops up the superoxide. Amyotophic Lateral Sclerosis (ALS), or Lou Gehrig’s Disease, is a crippling neuromuscular disease that usually attacks people between the ages of fifty and sixty. Strong evidence links ALS to mutations in the SOD1 gene. When the SOD gene is mutated, the enzyme is also mutated. The mutated enzyme loses its anti-oxidant effect. Inhibiting this enzyme could slow or stop the degeneration of nerve cells, which leads to the disruption of muscle control in ALS patients. As an enzyme, SOD has particular value as an antioxidant that can help to protect against cell destruction. It has the ability to neutralize superoxide, one of the most damaging free radical substances in nature. Like so many other protective compounds which naturally occur in the body, it decreases with age, making cells much more vulnerable to the oxidants which cause aging and disease. It occurs naturally in broccoli, Brussels's sprouts, wheat grass and in the majority of green plants.
This is my last blog for the semester because now it is time for Christmas Break. Even though this was the most challenging thing in Biology for me this year I think I understand enzymes pretty well now.
MERRY CHRISTMAS!
and
HAPPY NEW YEAR!
SEE ALL OF MY BIOLOGY FRIENDS NEXT YEAR IN 2011!
Thursday, December 16, 2010
Wednesday, December 15, 2010
PKU Webquest
Phenylketonuria is a long word! In my blog whenever I mean to say Phenylketonuria I am going to say PKU. To help understand this diesese we were given some questions and websites to look at. There was so much information on the websites that I couldn't put it all down, but I got as much as I could. Here are the links to the sites that Mr. Ludwig gave us.
PKU is a condition where phenylalanine builds up in the body. It is a very rare disease and is inherited. Phenylalanine is a building block of protein and is a natural substance. Mostly all babies get tested for PKU when they are just born. The test used is a heel prick test.
Your Genes, Your Health: Phenylketonuria
NSPKU Home Page
Texas Department of Health Genetic Disorders
Phenylketonuria - The Genetics
PKU is a condition where phenylalanine builds up in the body. It is a very rare disease and is inherited. Phenylalanine is a building block of protein and is a natural substance. Mostly all babies get tested for PKU when they are just born. The test used is a heel prick test.
To help us understand PKU better we were given questions to try and answer. They are kind of hard to understand at first but once you get to reading the sites it is easier.
1. What enzyme is most commonly defective in people with phenylketonuria?
The most enzyme that is most commonly defective in people with phenylketonuria is the PAH Enzyme.
2. What reaction does this enzyme catalyze? (What is the substrate and what product is produced?)
A person infected with PKU can get dangerously high phenylalanine in the brain. This can cause mental retardation and epilepsy.
3. Describe the symptoms of phenylketonuria?
The symptoms in children that have undetected PKU are smaller then normal head, lighter skin and hair then unaffected family members,
4. What causes the symptoms of PKU, the lack of a substance or the buildup of one?
The symptoms of PKU are caused by the lack of the enzyme to break down the amino acid which builds it up. The build up of the amino acid causes the symptoms. The state of high levels of phenylalanine is called Hyperphenylalaninaemia. (long word I know!) Hyperphenylalaninaemia can cause brain damage.
5. How common is phenylketonuria? How is it treated?
PKU is found in about 1 in 10,000 births in Caucasians and East Asians. There are some ethnic groups that have higher rates. For example PKU is extremely rare in Africans.
As soon as children are diagnosed they must keep a low- protein diet and stay on the diet as long as they possibly can. Most experts recommend that people infected with PKU diet for life. Phenylalanine is found in the protein part of food. This is why the treatment is to keep a low protein diet. Foods that are not in this diet are meat, cheese, poultry, eggs, and milk.
Photosynthesis, Cellular Respiration, and Energy
Sierra, Sidney, and I all worked on a glogster together. This was my first time using glogster and I really liked it. There will probably be future glogsters on my blog in the future. =) But for now here is our glogster on Photosynthesis, Cellular Respiration, and Energy.
Friday, December 10, 2010
Photosynthesis "Dry Lab"
So instead of doing an experiment and writing down the observations, this week we went backwards. We were given a set of observations and we had to write the procedure that went along with this experiment. It was kind of hard for me at first but then I looked at other peoples blogs and that helped me understand. So here is my Photosynthesis "Dry Lab."
Materials
Procedure
Observations:
Water plus BTB is blue-green.
Water is neutral. It changes to the color of the substance that is put in it.
Water plus BTB and an Aquarium Snail is yellow in light.
Animals respire (breath) and they let Carbon Dioxide out. Carbon dioxide in water produces carbonic acid. When there is acid in BTB and water it turns to yellow.
Water plus BTB plus elodea is blue-green in light.
Green plants respire. Then they photosynthesize and use the Carbon Dioxide. The plant keeps the water from acid it stays a neutral at the blue green color.
Water plus BTB plus a snail plus elodea is blue-green in light.
The plant and snail respire. But the plant photosynthesizes and uses the Carbon Dioxide so it turns to blue green and there is no carbonic acid.
Water plus BTB plus a snail plus elodea is yellow in dark.
The snail and plant respire. Since there is no light the plant can't photosynthesize. The carbon dioxide is still in the water so it stays an acid and the color stays yellow.
Conclusion:
When BTB is added to water it turns yellow because Carbon Dioxide and water mixed together make a carbonic acid. It stays blue with just water because water is a neutral.
Materials
- Distilled Water
- Bromothymol Blue (BTB)
- Aquarium Snail
- Elodea
- Large test tubes
- Light
- Dark space
Procedure
- Put 15 ml of water and 15 drops of BTB in a large test tube and let it sit for 3 hours under light. Record your observations.
- Put 15 ml of water, 15 drops of BTB, and an aquarium snail in a large test tube and let it sit for 3 hours under light. Record your observations.
- Put 15 ml of water, 15 drops of BTB, and a elodea (funny plant) in a large test tube and let it sit for 3 hours under light. Record your observations.
- Put 15ml of water, 15 drops of BTB, an aquarium snail, and an elodea in a large test tube in the light for 3 hours. Record your observations.
- Repeat Step 4 procedure but put it in the dark for three hours and let it sit. Record your observations.
Observations:
Water plus BTB is blue-green.
Water is neutral. It changes to the color of the substance that is put in it.
Water plus BTB and an Aquarium Snail is yellow in light.
Animals respire (breath) and they let Carbon Dioxide out. Carbon dioxide in water produces carbonic acid. When there is acid in BTB and water it turns to yellow.
Water plus BTB plus elodea is blue-green in light.
Green plants respire. Then they photosynthesize and use the Carbon Dioxide. The plant keeps the water from acid it stays a neutral at the blue green color.
Water plus BTB plus a snail plus elodea is blue-green in light.
The plant and snail respire. But the plant photosynthesizes and uses the Carbon Dioxide so it turns to blue green and there is no carbonic acid.
Water plus BTB plus a snail plus elodea is yellow in dark.
The snail and plant respire. Since there is no light the plant can't photosynthesize. The carbon dioxide is still in the water so it stays an acid and the color stays yellow.
Conclusion:
When BTB is added to water it turns yellow because Carbon Dioxide and water mixed together make a carbonic acid. It stays blue with just water because water is a neutral.
Subscribe to:
Posts (Atom)